Oxoacids of Sulphur
Oxoacids of Sulphur: Overview
This topic covers the concept of Oxoacids of Sulphur and Their Structures.
Important Questions on Oxoacids of Sulphur
The general formula for polythionic acid series is , where to What is the value of for pentathionic acid acid?
The acid, which does not contain peroxy linkage, is
The structure of sulphurous acid is:
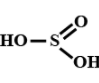
Which of the following are peroxo acids of sulphur:-
In , the oxidation state of is _______
Which of the following oxoacid of sulphur has linkage?
Name the different oxyacids of sulphur? and draw their structures.
Which of the following are peroxoacids of sulphur?
Which among and are peroxy acids?
the with and mark the appropriate choice.
| , dibasic | |||
| , dibasic | |||
| , monobasic | |||
| , dibasic |
The acid in which O - O bonding is presents is
Which of the following represents the correct order of decreasing number of bonds?
In which pair of ions do both the species contain bond?
Which property of makes it useful in photography?
Which one of the following is not a true "Per acid"?
In which of the following molecules there is no bond?
Which of the following molecules has as bond ?
The oxidation states of S-atoms in Marshall's and Caro's acid are
Identify the correct order of increasing number of bonds in structures of the following molecules.
When sulphur is boiled with Na2SO3, a compound (A) is produced. (A) with excess of AgNO3 solution gives a compound (B) which is water soluble and produces a black coloured sulphide (C). Compounds (A), (B) and (C) will be respectively :
